Children and adults with NF1 are prone to develop brain tumors. In children, most of these tumors are low-grade gliomas involving the optic nerve, called an optic pathway glioma (OPG), while adults can develop more aggressive (malignant) gliomas.
Using a variety of different methods and approaches, investigators in the Washington University NF Center are focused on creating small animal and tissue culture models of NF1-associated brain tumors. These preclinical models are employed to discover new anti-cancer drugs and evaluate their safety and efficacy prior to clinical trials in people with NF1.
- Cellular origins of brain tumors
- Role of immune cells in brain tumor formation and growth
- Role of nerves in brain tumor development
- Identification and preclinical evaluation of new brain tumor therapies
Cellular origins of brain tumors
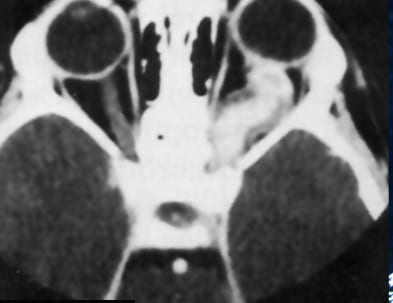
Research over the past decade has shown that brain tumors arise from a number of different types of cells in the brain. Determining where these tumors come from in children and adults with NF1 may yield new insights into how best to prevent their formation.
To understand the cellular origins of NF1-associated gliomas, Dr. David Gutmann, Dr. Nicole Brossier, Dr. Corina Anastasaki and their colleagues are using novel mouse strains and patient-derived tumor models. These studies aim to identify which cells in the developing brain give rise to low-grade gliomas in children and malignant brain tumors in young adults with NF1.
Role of immune cells in brain tumor formation and growth
Brain tumors contain both cancerous and non-cancerous cells, specifically immune system cells called T lymphocytes (T cells) and monocytes (microglia). Studies in the Washington University NF Center have revealed that these immune cells play critical roles in the development and growth of optic gliomas.
Studies ongoing in Dr. Gutmann’s laboratory are focused on understanding how these immune cells support brain tumor growth, how they are recruited into the developing tumor, and how they could be modified using immune therapies to block optic glioma development and growth.
Role of nerve cells in brain tumor formation and growth
Brain tumors form and grow in an environment rich in nerves. While not conventionally thought to control tumor development and progression, recent studies have revealed that nerve cells are important drivers of optic glioma initiation and continued growth.
Studies in the laboratories of Dr. Gutmann, Dr. Anastasaki and Dr. Pan are focused on understanding how mutations in the NF1 gene cause nerve cells (neurons) to be hyperexcitable, defining the mechanisms by which nerves control tumor growth, and identifying therapies that safely block neuron activity-driven tumor expansion.
Identification and preclinical evaluation of new brain tumor therapies
After the identification of the NF1 gene and its protein (neurofibromin), several promising drugs have been identified that are currently being evaluated as potential therapies for children and adults with NF1. However, these treatments do not work in all individuals and are often associated with serious side effects. To provide alternatives to our currently available therapies, investigators in the Washington University NF Center are identifying and evaluating new drugs for the treatment of optic glioma, brain tumors, neurofibromas, and malignant peripheral nerve sheath tumors using mice and patient-derived tumor specimens.
These studies are designed to rapidly test the most promising candidate drugs prior to their evaluation in children and adults with NF1. Additional compounds are also currently being evaluated for their ability restore or prevent vision loss from NF1-OPG.